
 |
|||||
|
|
|||||
|
|||||||||||||||||||||||||||||||||||
 |
|||||||||||||||||||||||||||||||||||
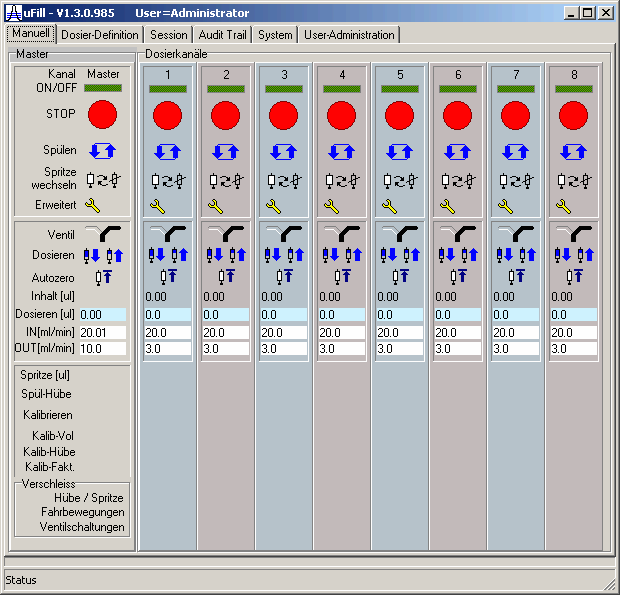
| Each of the eight syringe pumps has its representation on the manual tab. The design is adapted to the one of audio mixing desks which exactly fits the same purpose. The current valve position of the syringe pumps is shown. All functions of the syringes can be controled and surveyed from this screen, like: Online/Offline-switch, emergency stop, rinsing, change of syringe, valve switching, manual dosage, semiautomatic validation/calibration and monitoring of the service parameters to exchange spare parts according to the servicing instructions of the manufacturer. | |||||||||||||||||||||||||||||||||||
 |
|||||||||||||||||||||||||||||||||||
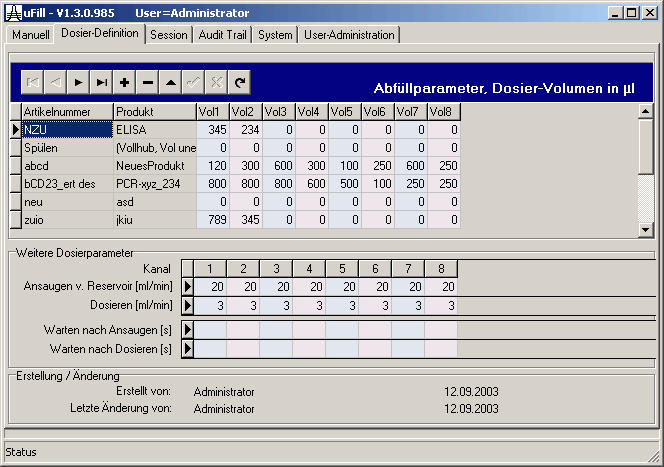
| Definition of a batch process like volumes for each channel can be done only by administrator or process designer. The data are stored in a database. According to 21CFR11 each change of a process definition is monitored. | |||||||||||||||||||||||||||||||||||
 |
|||||||||||||||||||||||||||||||||||
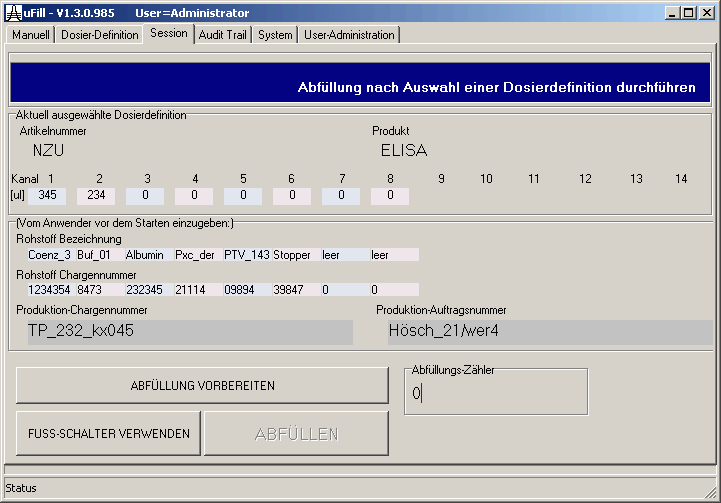
| The separate dosages initially have been semiautomatic with the help of a footswitch. | |||||||||||||||||||||||||||||||||||
 |
|||||||||||||||||||||||||||||||||||
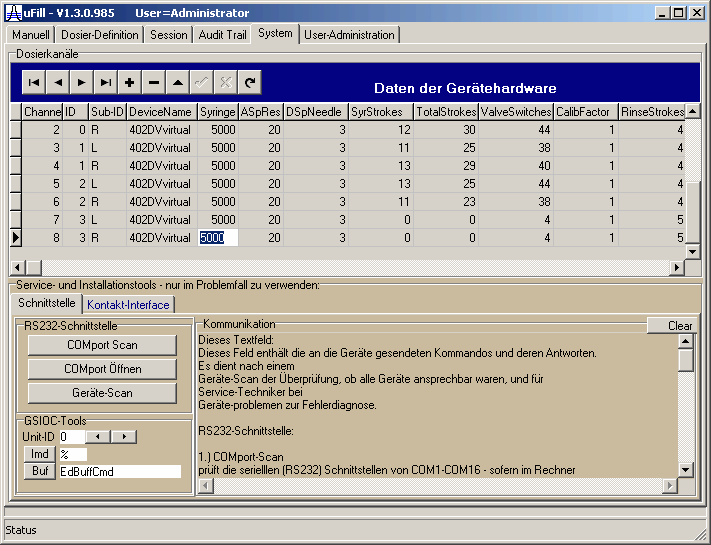
| The system setup also is stored in the database and can be changed only by an administrator. It contains tools to detect hardware problems. | |||||||||||||||||||||||||||||||||||
 |
|||||||||||||||||||||||||||||||||||
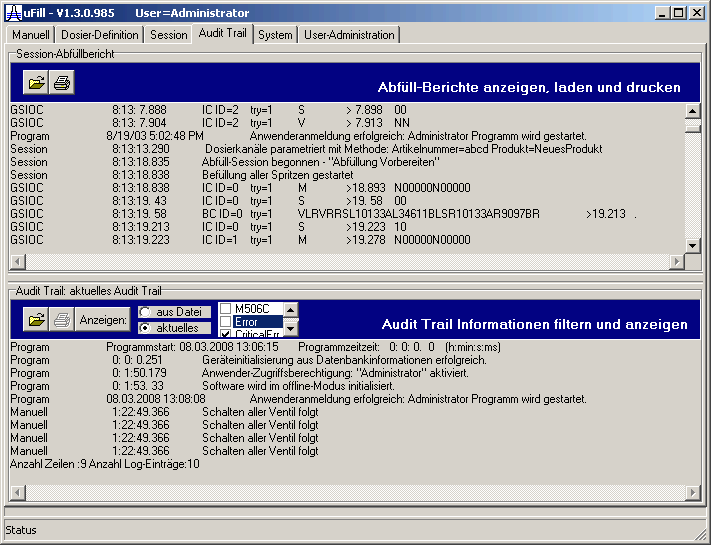
| The 21CFR11 compliant audit trail log saves all device commands which pass the RS232 port, as well as messages from higher program architecture layers, like: communication protocol layer, device objects, process, and user interactions.
The audit trail can be filtered for: Critical and uncritical errors and the different architecture layers. An error statistic at the end of an audit trail sums up all errors, the device command repetitions (done in case of a communication error), as well as the successful commands and device requests. With this protocol it can be proven, that the devices did what they should have done. |
|||||||||||||||||||||||||||||||||||